Antibody drugs are one of the most important biologic therapeutics. The downstream purification process is one of the key links in the production process, accounting for 50% of the production cost. The commonly used antibody drug purification is mainly classical three-step chromatography. Affinity chromatography is mainly used to capture the target antibody. Anionic chromatography and cationic chromatography are used to remove the host cell residual protein, host cell DNA and other impurities to further improve the purity of antibody.
arProtein A Focurose HR affinity chromatography resin has been launched by VDOBIOTECH for the separation and purification of ascites, cell culture supernatant, serum-derived monoclonal antibody, polyclonal antibody and Fc label protein. The resin is based on high-strength cross-linked agarose, which is independently fermented by the company. It has the advantages of high pressure resistance, excellent alkaline stability and high capacity, which can meet the demand of antibody drug customers for purified antibodies.
The characteristic of arProtein A Focurose HR:
1.High rigidity agarose matrix
2.Particle size uniformity:75µm±5µm
3.Excellent alkaline stability:0.1-0.5M NaOH
4.Stabilized in all commonly used buffers
5.High velocity and high pressure resistance
High binding capacity:Under 5min retention time, dynamic binding capacity is more than 60mg/mL.
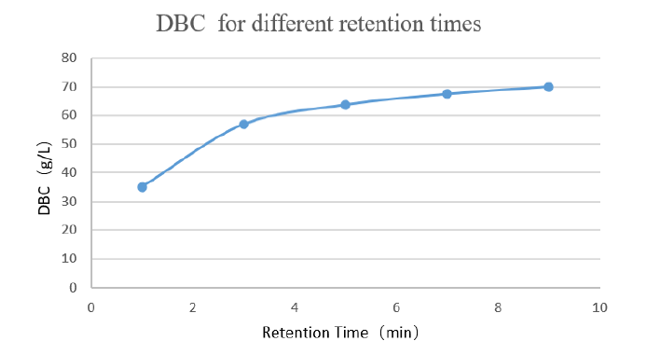
Figure 1 Dynamic load with different retention times
Excellent pressure-flow curve:
In HK 16mm/40cm column, bed height 26 cm, when 500 cm/h velocity operating pressure is no more than 0.25MPa.
Under the same column high and linear velocity, our developed columns back pressure can be comparable to production level column:
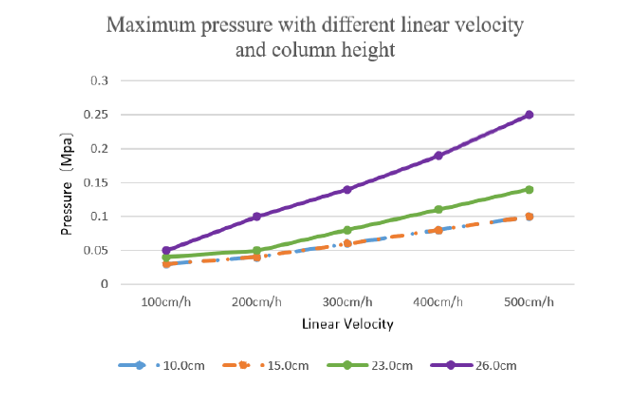
Figure 2 Maximum pressure with different linear velocity and column height
High alkaline stability
Static alkali resistance:The filler was soaked in 0.1M, 0.5M and 1.0M NaOH solution for a certain time, cleaned with pure water to neutral, and then pre-loaded 1ml column to test the capacity. As shown in figure 3, the DBC capacity was not affected by soaking in 0.1M NaOH for 1440h. After 168h soaking in 0.5M NaOH and 1M NaOH, DBC capacity decreased within 20%, which proves that the filler has good alkaline stability.
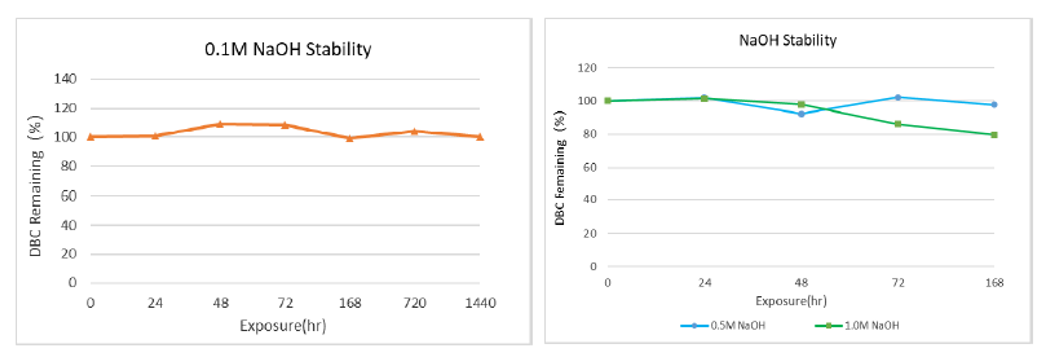
Figure 3 arProtein A Focurose HR static alkali resistance
Dynamic alkali resistance:The capacity of arProteinA Focurose HR was tested by in-place cleaning (CIP) with 0.1M and 0.5M NaOH for 15min. The results are shown in figure 4. The DBC for 200 cycles of 0.1M NaOH remains basically unchanged, while the DBC for 100 cycles of 0.5M NaOH remains above 80% of the initial value. The resin has good alkaline resistance, improves the service life in the process of antibody production, and greatly reduces the production cost.
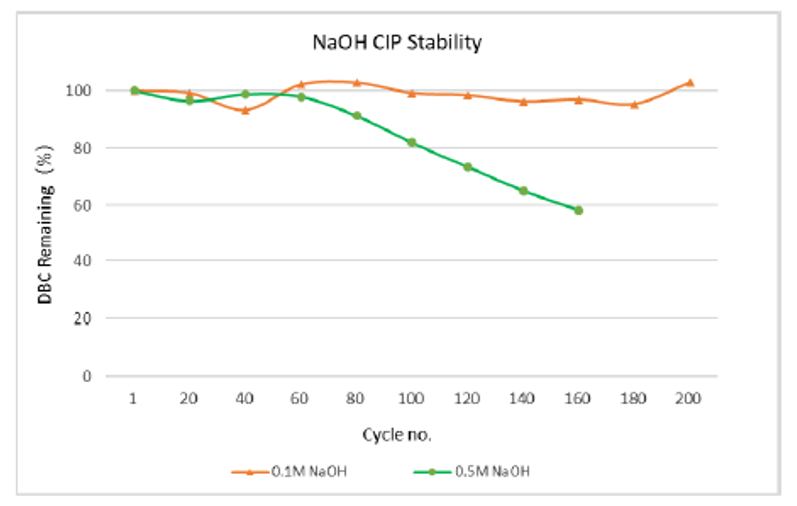
Figure 4 arProtein A Focurose HR static alkali resistance
Lifetime verification
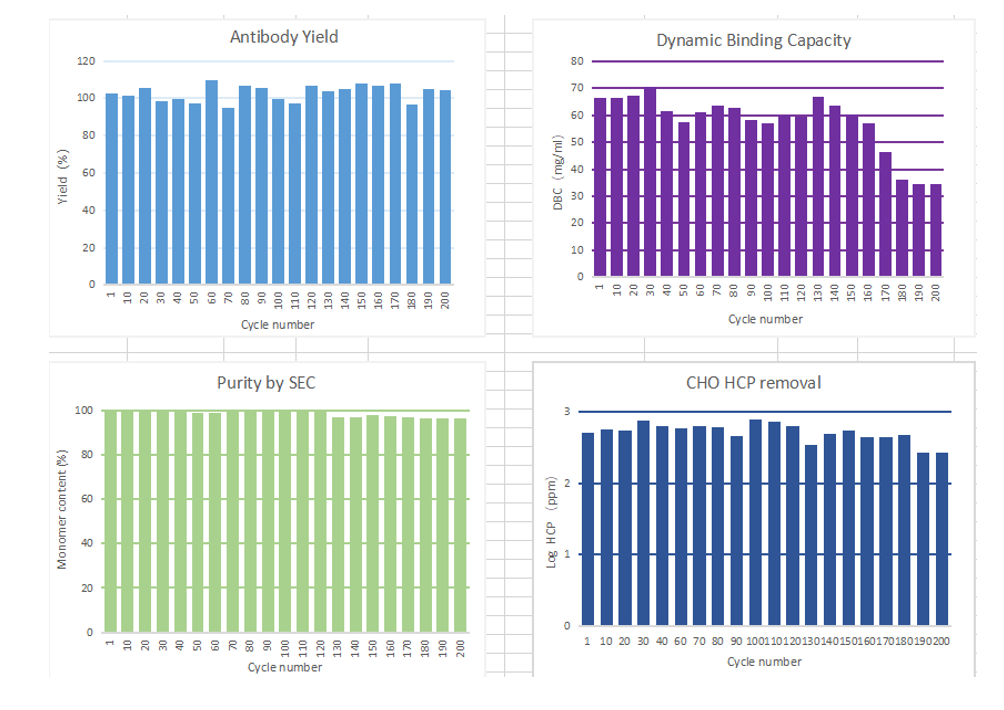
In the 200 times of monoclonal antibody lifetime verification, all the performance indexes could meet most of the requirements of antibody production: the recovery rate of antibody did not decrease significantly in the whole life process, and the decrease range was basically within 10%. The purity by SEC is maintained above 95%, with strong stability;Dynamic binding capacity was maintained at about 60mg/mL, and the decrease rate was within 15% within 160 cycles; The host cell proteins were all less than 800ppm.
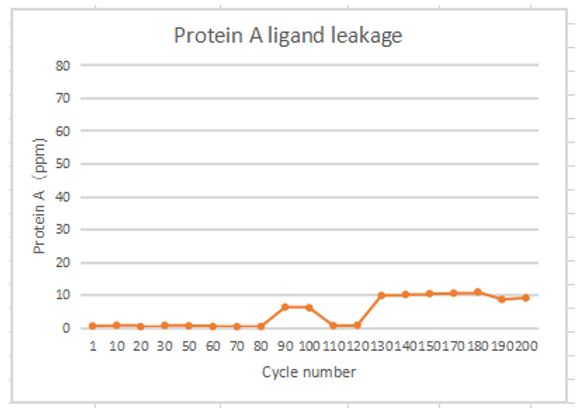
In the 200 times of monoclonal antibody lifetime verification, Protein A ligand residues were all less than 10ppm. And there was almost no ligand shedding in 80 times.
VDO has established a fully automatic large-scale production line to meet the GMP standard requirements and to ensure that arProtein A Focurose HR without animal contamination. At the same time, we can provide customers with a variety of regulatory declaration support documents, product specifications, COA documents, RSF regulatory support documents, SDS/MSDS documents, no animal origin description, ISO 9001 certification.
Order Information
Product | Specification | Art.No. |
arProtein A Focurose HR | 1mL prepacked column | HQ320827001E |
5mL prepacked column | HQ320827005E | |
25mL | HQ320827025M | |
100mL | HQ320827100M | |
500mL | HQ320827500M | |
1L | HQ320827001L | |
5L | HQ320827005L | |
20L | HQ320827020L |


