In order to better serve the chromatographic purification customer group, VDOBIOTECH has been comprehensively upgraded from the research and development production control, quality testing and application development of chromatography resins.
1. R&D design
As an important development direction for chromatographic resin in the future, affinity chromatography is centered on microspheres as carriers and ligands that bind to target proteins. Our team has successfully developed a variety of affinity chromatography resin for antibodies, viruses and a variety of biological nanoparticles.
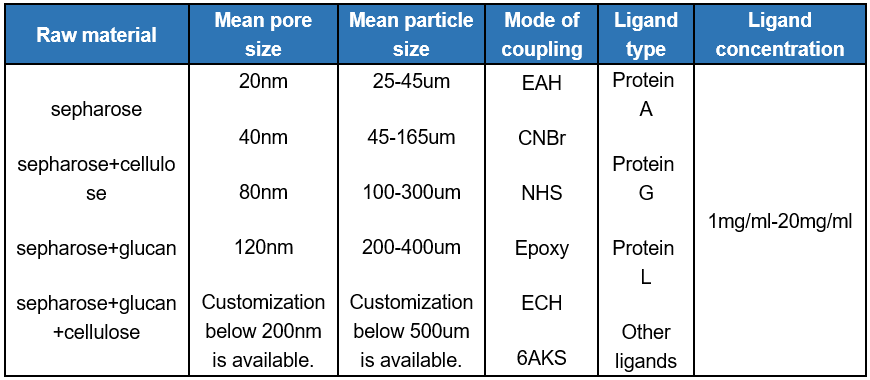
At the same time, the research and development team can adjust the pore size, particle size, ligand type and other parameters of the product according to the requirements of the purification process of customers, to provide customers with professional and customized chromatographic resin. And we can combine with purification process development services to meet the individual needs of customers.
2. Production control
VDOBIOTECH have established a fully automatic large-scale production line and actively practice the concept of lean production. The current annual production capacity can reach 100t and single batch capacity can achieve 1200L. The whole process is equipped with DCS distributed control system, which ensures the continuous stability and safe and efficient production of chromatographic resin, and effectively avoids the difference between batches caused by the production process. GMP clean workshop ensures that chromatographic resin is free from external impurity contamination and quality safety.

At the same time, VDOBIOTECH is also developing a new generation of chromatographic resin production process for different materials and different methods. After the implementation of the new process, it is expected to achieve better performance while reducing costs and improving environmental protection in the future.
3. Quality Control
VDOBIOTECH has established a strict quality management system, which completely covers the whole process of product planning, design and development, product verification, product realization, transportation, process service and after-sales service. We establish a professional quality team (QA, QC, QR, QE, QU) and total quality control standards (particle size distribution, linear flow rate, pressure resistance, microbial residue, non-specific adsorption, ligand concentration, dynamic capacity, solvent residue, ligand shedding, etc.) for the chromatographic resin product line.

Also we can provide customers with a variety of regulatory support documents, such as COA, RSF, ISO 9001,etc.
4. Application development
In order to respond to the process development needs of customers more quickly and timely, VDOBIOTECH has set up a new chromatographic resin application center in Suzhou BioBAY. The laboratory is equipped with a powerful application development team, a number of high-end purification and analysis equipments.

At present, we have established several platform purification processes, including Antibody platform purification process, Virus platform purification process, Plasmid platform purification process, Purification process of mRNA platform.

