Affinity resins were established and developed based on the principle of specific adsorption between biomolecules and other ligand molecules (e.g. antigen and antibody, enzyme and substrate, hormone and receptor, complementary chain in nucleicacid, polysaccharide and protein complex, etc.). The purification of target molecules is achieved by specific adsorption between the ligand on the medium and the target molecule. Due to this specific force, affinity resins are characterized by high selectivity and high activity recovery.
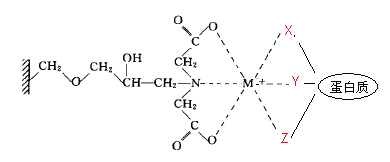
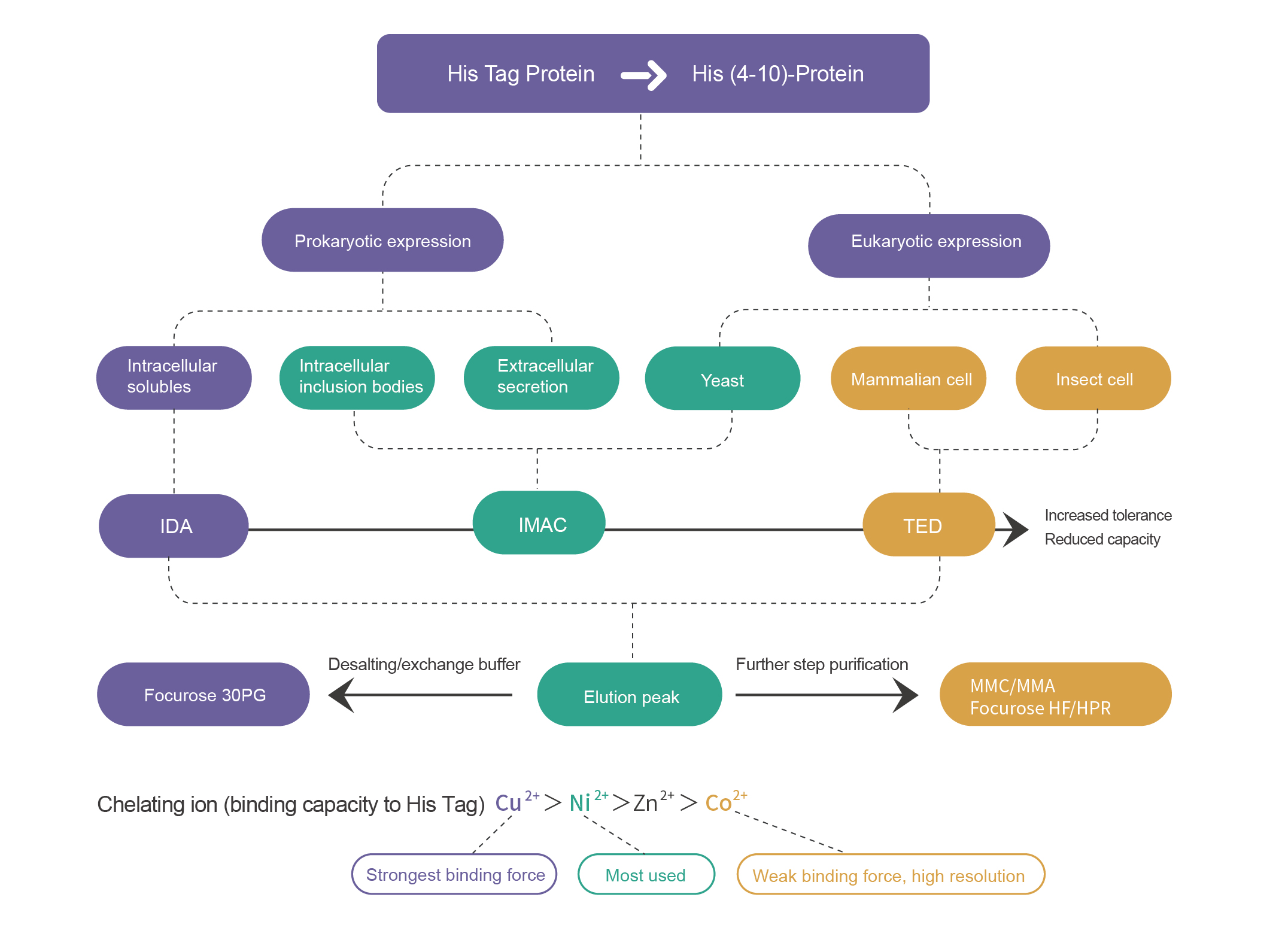
Transition state metal ions (Cu >Ni >Zn >Co ) can bind to electron donors, such as N, S, O and other atoms with coordination bonds. The remaining empty orbitals on the metal ions are ligand sites for electron donors, which will be occupied by water molecules or anions in solution. When the amino acid residues (His) on the protein surface are strongly bound to metal ions, the powered atoms of the amino acid residues will bind to the metal ions to form a complex, replacing the previously bound water molecules or anions, thus enabling the protein molecules to bind to the solid surface. His-tagged proteins with His and mediator binding are purified by selecting different metal ligands depending on the affinity of the metal ligands due to the different types, numbers, positions and spatial conformations of the amino acids on the protein surface.