Hydrophobic Interaction Resins

Hydrophobic interaction resins separates proteins based on differences in hydrophobicity, i.e., based on reversible interactions between proteins and hydrophobic groups on the surface of hydrophobic interaction resins. Hydrophobicity isenhanced at high ionic strengths and therefore binding in a high ionic strength environment is usually eluted by reducing the ionic strength. The unique adsorption separation mode makes hydrophobic interaction resins an ideal purification method after ammonium sulfate chromatography or after ion exchange high salt elution.
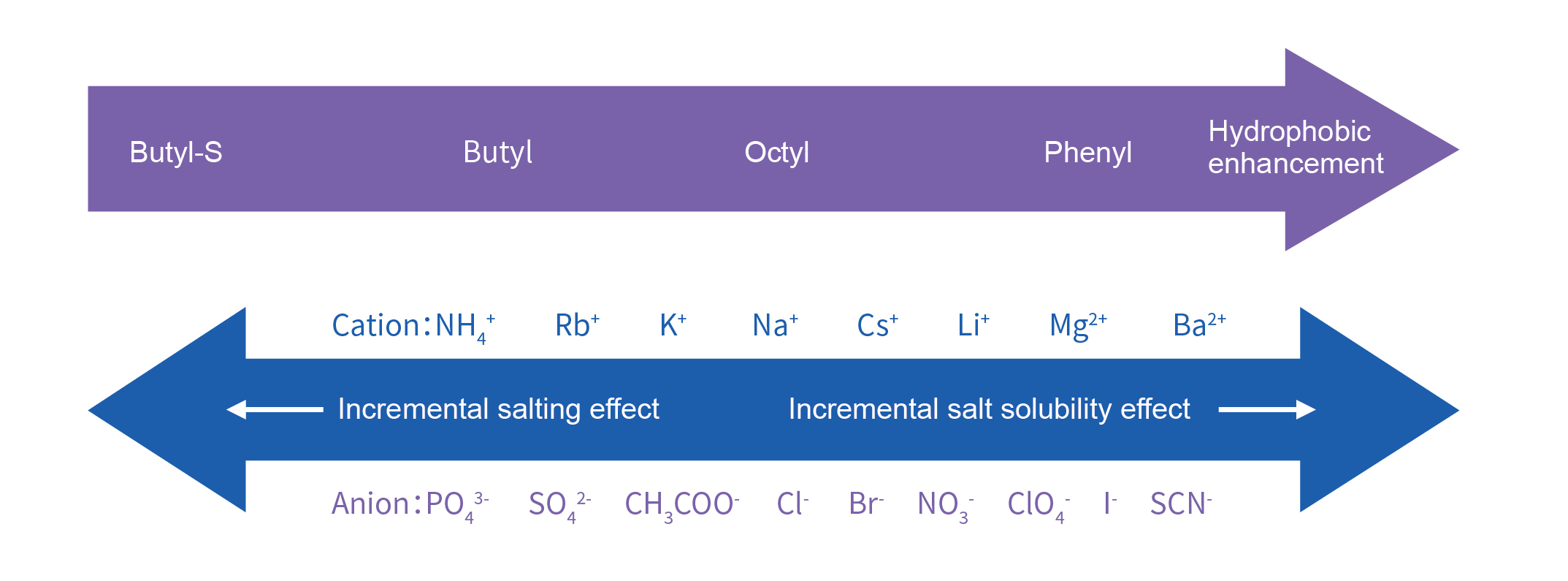
Hydrophobic Interaction Resins
Hydrophobic Chromatography Media
Phenyl Focurose FF(HS)
Phenyl Focurose FF(LS)


